Experiment 15 quantitative preparation of potassium chloride – Embark on a scientific journey with Experiment 15: Quantitative Preparation of Potassium Chloride. This meticulously crafted experiment delves into the fundamental principles of quantitative chemistry, guiding you through the precise steps of preparing potassium chloride in a controlled and measurable manner.
As we unravel the intricacies of this experiment, we will uncover the significance of accurate measurements, explore the diverse applications of potassium chloride, and delve into the safety precautions essential for a successful and hazard-free laboratory experience.
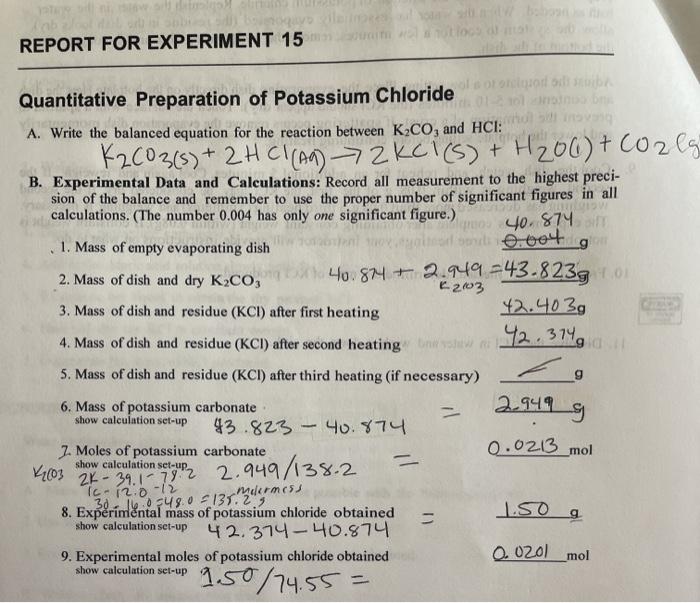
Quantitative Preparation of Potassium Chloride

The quantitative preparation of potassium chloride (KCl) is a fundamental experiment in chemistry that demonstrates the principles of quantitative analysis and stoichiometry. It involves the controlled reaction of potassium hydroxide (KOH) and hydrochloric acid (HCl) to form KCl.
Quantitative analysis plays a crucial role in various scientific disciplines, including chemistry, biology, and environmental science. It enables researchers to accurately determine the concentration or amount of a substance in a sample. This experiment provides a hands-on experience in applying quantitative methods to prepare a known quantity of KCl.
Materials and Equipment
- Potassium hydroxide (KOH) pellets
- Hydrochloric acid (HCl) solution
- Analytical balance
- Burette
- Erlenmeyer flask
- Magnetic stirrer and stir bar
- Filter paper and funnel
- Drying oven
The analytical balance is used to accurately weigh the KOH pellets, while the burette is used to dispense the HCl solution. The Erlenmeyer flask serves as the reaction vessel, and the magnetic stirrer ensures thorough mixing of the reactants. Filter paper and a funnel are used to filter the KCl crystals, and the drying oven is used to remove any remaining moisture.
Procedure
- Weigh a known mass of KOH pellets and dissolve them in distilled water in an Erlenmeyer flask.
- Set up the burette with the HCl solution.
- Slowly add the HCl solution to the KOH solution while stirring constantly.
- Continue adding HCl until the equivalence point is reached, as indicated by a pH meter or a suitable indicator.
- Filter the KCl crystals using a filter paper and funnel.
- Wash the crystals thoroughly with distilled water to remove any impurities.
- Dry the KCl crystals in a drying oven at 110°C for several hours.
- Weigh the dried KCl crystals to determine the yield.
The equivalence point is the point at which the moles of HCl added are equal to the moles of KOH initially present. At this point, all of the KOH has reacted to form KCl.
Calculations
The quantitative yield of KCl is calculated using the following formula:
Quantitative Yield = (Actual Mass of KCl / Theoretical Mass of KCl) x 100%
The theoretical mass of KCl is calculated using the stoichiometry of the reaction:
KOH + HCl → KCl + H2O
From the balanced equation, 1 mole of KOH reacts with 1 mole of HCl to form 1 mole of KCl. Therefore, the theoretical mass of KCl can be calculated using the mass of KOH used and the molar mass of KCl.
Results and Discussion: Experiment 15 Quantitative Preparation Of Potassium Chloride

The experimental results should be presented in a table or bullet points. The table should include the mass of KOH used, the volume of HCl added, and the mass of KCl obtained. The accuracy and precision of the results should be discussed, and any sources of error should be identified.
The quantitative yield of KCl should be calculated using the formula provided in the previous section. The yield should be within an acceptable range, and any significant deviations from the theoretical yield should be discussed.
Applications
Potassium chloride has a wide range of applications in various fields, including:
- Medicine:KCl is used as an electrolyte replacement in intravenous fluids and as a treatment for hypokalaemia (low potassium levels in the blood).
- Agriculture:KCl is used as a fertilizer to provide potassium to plants, which is essential for plant growth and development.
- Industry:KCl is used in the production of glass, ceramics, and other industrial products.
Potassium chloride’s unique properties, such as its high solubility, stability, and low toxicity, make it suitable for these applications.
Safety Precautions
The following safety precautions should be taken when conducting this experiment:
- Wear appropriate personal protective equipment, including gloves, safety glasses, and a lab coat.
- Handle KOH and HCl with care, as they are corrosive substances.
- Conduct the experiment in a well-ventilated area.
- Dispose of all chemicals and waste materials properly.
By following these safety precautions, the risk of accidents or injuries can be minimized.
Q&A
What is the purpose of Experiment 15: Quantitative Preparation of Potassium Chloride?
Experiment 15 aims to provide a hands-on experience in quantitative chemistry, demonstrating the precise preparation of potassium chloride and the determination of its quantitative yield.
Why is quantitative preparation important in chemistry?
Quantitative preparation ensures accurate and reproducible results in chemical experiments, allowing scientists to control the stoichiometry of reactions and obtain reliable data for analysis and interpretation.
What safety precautions should be taken while conducting Experiment 15?
Proper laboratory attire, including gloves, safety goggles, and a lab coat, should be worn throughout the experiment. Careful handling of chemicals and proper waste disposal are crucial to minimize potential hazards.